
Chemical Production
Salt separation process - Mine water project
Waste source:
In the process of coal mining, the contact of groundwater, coal and rock, with the influence of human activities, leads to a series of physical, chemical and biochemical reactions, so the water quality has significant characteristics about coal industry: the suspended matter in mine water is much higher than the surface water, and the suspended matters have small size, light weight, slow sedimentation and poor coagulation effect. The mine water also contains organic pollutants such as organic waste oil and emulsified oil. The total ion content in mine water is much higher than the surface water, and most of the ions are sulfate ions.
Separation process:
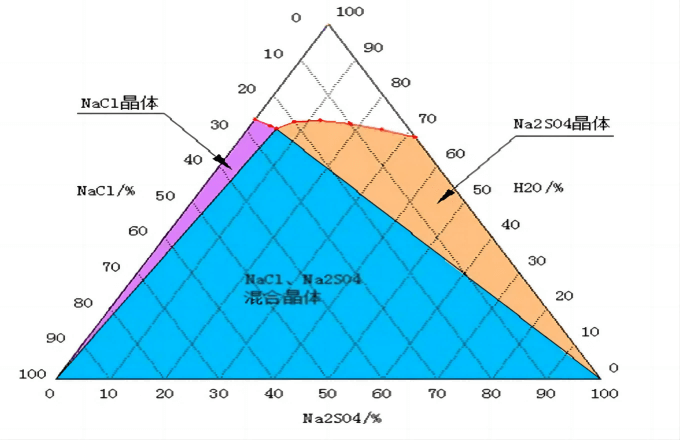
Based on the difference in the solubility of sodium sulfate and sodium chloride at low temperature, the water-salt phase diagram of “Na2SO4-NaCl-H2O” is used to separate. The industrial-grade sodium sulfate and sodium chloride products obtained can be sold, so as to reduce the cost of solid waste disposal and create value for enterprises.

Salt separation process - coal chemical industry project
Generation of Coal chemical industry wastewater:
It is the industrial wastewater produced in the production process of coal chemical industry. Coal is the raw material of coal chemical production. Through coal coking, coal gasification, coal liquefaction, tar chemical industry, calcium carbide acetylene chemical industry, chemical products recycling and other chemical production process, the coal is transferred into gaseous, liquid, solid products and a variety of chemical products. The wastewater from the production process mainly includes coking, gasification and liquefaction wastewater.
Separation process:
Because coking wastewater mainly contains sodium chloride, sodium sulfate, and the proportion of the two salts is similar. If directly adopted evaporation and freezing process, the rate of product recovery is low, and the impurity rate is higher. Therefore, coking wastewater generally needs nanofiltration first, freshwater side basically only contains sodium chloride, concentrated water side mainly contains sodium sulfate, also contains part of sodium chloride. After nanofiltration, respectively equipped with evaporator to process, recycling sodium sulfate, sodium chloride respectively.
Salt separation process - fly ash industry project
Generation of fly ash:
Due to the oxidation, pyrolysis and combustion of substances at a high temperature, and forms solid particles. the main components of some fly ash are sodium chloride and potassium chloride. Because both of them are soluble, they cannot be directly buried. Therefore, the two kinds of salt should be separated to make industrial products, especially the potassium salt, which has high recycling value.
Different solubility:
The solubility of potassium chloride is greater than that of sodium chloride. Meanwhile, the solubility of potassium chloride increases as the temperature increases, while the solubility of sodium chloride does not change much with the temperature.
Separation process:
Under different temperature conditions, the solubility ratio of sodium chloride and potassium chloride is different. Through the conversion of different evaporation temperatures, corresponding to the saturation states of the two salts, sodium chloride and potassium chloride are precipitated respectively, that is, sodium chloride can be obtained at high temperature and potassium chloride is at low temperature.




