
Spent Battery Recycling
Resource recovery of spent batteries
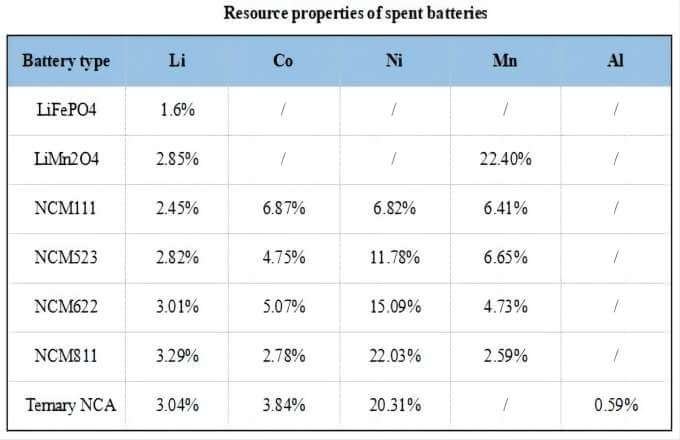
Power lithium batteries contain a large number of rare earth elements that have high economic value and non-ferrous metals such as cobalt, lithium, nickel, copper, aluminum and other metal content that is higher than ore.
The recycling of spent power batteries not only has important economic value, but also can alleviate the shortage of mineral resources, which is of great significance to reduce the battery production cost and promote the development of electric vehicle industry.
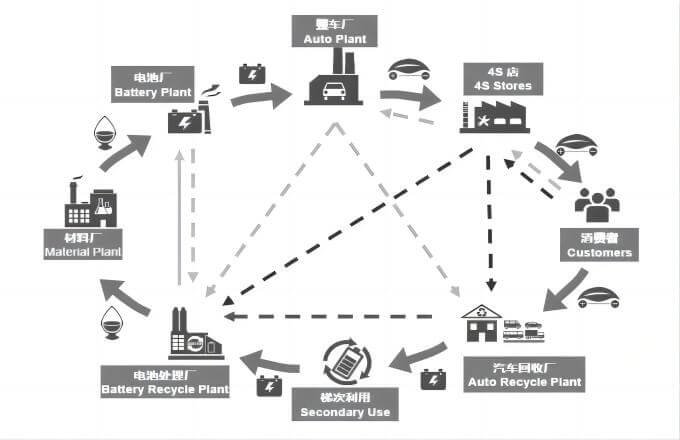
Methods & Technologies of Battery recovery
There are two ways of power battery recycling:
- Cascade utilization
- Disassembly recycling
Lithium iron phosphate is suitable for cascade utilization at first and then disassembly and recycling; ternary battery is suitable for direct disassembly and recycling.
When the normal discharge at 1C times rate, the capacity decay rate of the lithium iron phosphate battery is much less than that of the ternary battery. When the battery capacity decays to 80%, the lithium iron phosphate battery retired from the car still can be recycled for many times, so it has a high value of cascade utilization. The raw materials of ternary battery contain high-value metal elements, and its disassembly and recovery value is much higher than that of lithium iron phosphate battery.
Disassembly & recovery process of power battery
Physical recovery:
The internal components of waste power battery such as electrode active substance, fluid and battery shell, through a series of means such as crushing, screening, magnetic separation, fine crushing and classification, to obtain valuable products and then go to the next recycling process. The core process is the material repair after crushing and screening, it is a relatively pure physical process.
Hydro-metallurgy technology:
After disassembly and pretreatment, dissolve the waste batteries in the acid and alkali solution to extract the valuable metals, and then extract the remaining by means of ion exchange method and electrodeposition. The core of the wet process is to leach and extract the electrode powder.
Fire metallurgy:
Peel off the battery shell and mix the battery core with coke and limestone, through reduction roasting, to obtain the carbon alloy made up of metal lithium, cobalt, nickel, aluminum and others; the fluorine and phosphorus in the electrolyte are solidified in the slag and can be used for building materials or concrete additives. Then the further processing is conducted, and the whole process is completed at high temperature. The core process of fire metallurgy is pyrolysis at high temperature to obtain metal oxides.


solutions of spent power batteries
- Removal of manganese and the electrolytic manganese dioxide process
Through the electrolysis to generate oxygen radicals and oxygen, to oxidize divalent manganese, forming manganese dioxide. Although this method has higher energy consumption than the past, but its efficiency is high, the electrolytic manganese dioxide produced by it can also be sold as a high value-added product, and still has many advantages compared with the previous products with low purity.
- High COD removal
The method of carbon adsorption and Fenton oxidation is used to remove the organics in the wastewater. Reduce the complex treatment process of wastewater, without using the biological pool, can treat a large amount of wastewater efficiently, making the wastewater reach the standard of MVR evaporation system.
- Evaporation and Crystallization
MVR is used to evaporate the sodium sulfate solution without organics. The sodium sulfate crystallized by evaporation is dried by the dryer to get the anhydrous sodium sulfate salt, which can be sold to generate economic value.



