
Lithium Brine Extraction
Technologies & applications of lithium extraction
Precipitation method:
The precipitation method is mainly divided into two parts:
- Separation and flotation of potassium chloride
- Carbonization preparation of lithium carbonate
The specific process is, the brine that NaCl is removed by precipitation evaporates and crystallizes to separate KCl, after flotation drying, KCl is obtained, and let the rest of the brine in the lithium evaporation pond, enriching Li concentration to 6%, with organic solvent to remove boron, adding soda and lime to remove Mg, then adding soda again can get 99% purity of lithium carbonate.
Ion-exchange adsorption method:
The principle of ion exchange adsorption method is to adopt selective adsorbent to absorb Li+, and after Li+ is eluted, use nanofiltration membrane to remove magnesium under acidic conditions. After RO concentration and evaporation, high-lithium liquid is obtained. Finally precipitate and filter to obtain lithium carbonate.

Solar pond method:
Solar pond is composed of upper, middle and lower layers, namely upper troposphere (UCZ), non-troposphere (NCZ) and lower troposphere (LCZ).
The components of upper troposphere is fresh water, its temperature approaches the ambient temperature and protects the lower layer; the components of lower troposphere is saturated salt solution, which has the function of heat absorption and heat storage; the concentration of non-troposphere increases with the depth of the pool; utilize the refractive index of fresh water and the brine, the heat can be stored in the brine of the bottom. Because the solubility of other salts except lithium carbonate in the carbonate brine increases with the increase of temperature, the solubility of lithium carbonate decreases with the increase of temperature, when the temperature of lower troposphere (LCZ) increases, lithium carbonate will be enriched at the bottom of the pool, while other salts are difficult to precipitate.
Solvent extraction method:
Lithium ions in salt lake brine can be extracted by solvent extraction method, its principle is, to mix the brine with the organic solvent whose density is not less than water and not dissolved with the brine, in the physical dissolution, separation or chemical reaction, the brine component is extracted into the organic phase, and then extract the aqueous phase from the organic solvent. At present, common Li extraction systems include neutral TBP/FeCl3/MIBK extraction system, crown ether compounds, β-diketone, ionic liquids, etc.
Extraction method is suitable for salt lake with high magnesium-lithium ratio. But the toxic and harmful problems of organic solvents lead to the increase of environmental protection cost, so this extraction method is not used on a large scale, and the production capacity is small.


Membrane separation method:
- Electrodialysis membrane
- Nanofiltration membrane
Electrodialysis membrane technology firstly used in seawater desalination. In the early of 21st century, it began to be used for the separation of magnesium lithium in salt lake brine. Its technical principle is, the use of alternating cation and anion exchange membrane, cation under the action of electric field goes through the cation exchange membrane, and anion passes through anion exchange membrane to the electrode, so monovalent cation (such as Li+, Na+, K+) transfers to the concentration chamber, but divalent cation (such as Ca2+, Mg2+) is blocked, staying in the desalination chamber, to achieve the purpose of magnesium lithium separation.
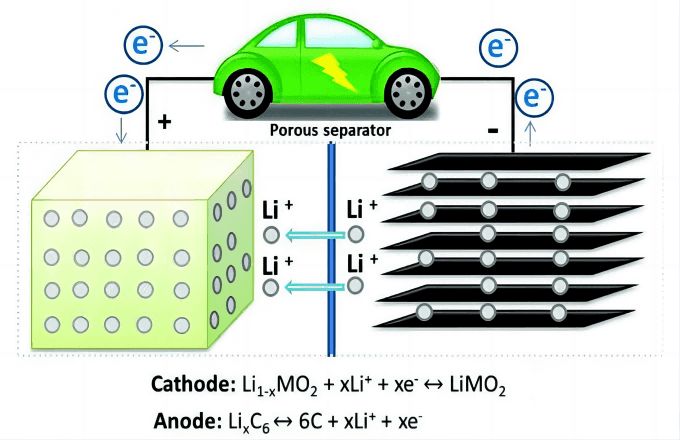
Electrochemical method:
The electrochemical lithium extraction method is developed according to the working principle of lithium battery. By controlling the potential to realize the embedding and stripping of Li+ in the electrode material, it can avoid the dissolution of the manganese and titanium ion sieve materials in the acid desorption process. Electrochemical method can significantly improve the operation cycle of adsorbent, and is suitable for lithium extraction in different brine systems. This method has not been industrialized, and the focus of R&D is on the selection and improvement of electrode materials.
Calcination and leaching method:
The principle of lithium extraction by calcination leaching is that lithium magnesium oxide and lithium magnesium carbonate are not soluble in water, and leaching magnesium oxide with water can achieve the purpose of lithium magnesium separation. The brine after acidification is concentrated to obtain magnesium chloride, after spray drying to get dihydrate magnesium chloride, and then calcine at 700~900℃ high temperature to get anhydrous lithium magnesium oxide mixture, add high purity water can leaching lithium, then by adding Ca (OH) 2 and Na2CO3 to remove calcium, magnesium and other impurities. Calcination leaching method is one of the earliest technical process to realize industrialization, but the energy consumption is large and toxic and harmful gases will be produced, resulting in serious environmental pollution.




