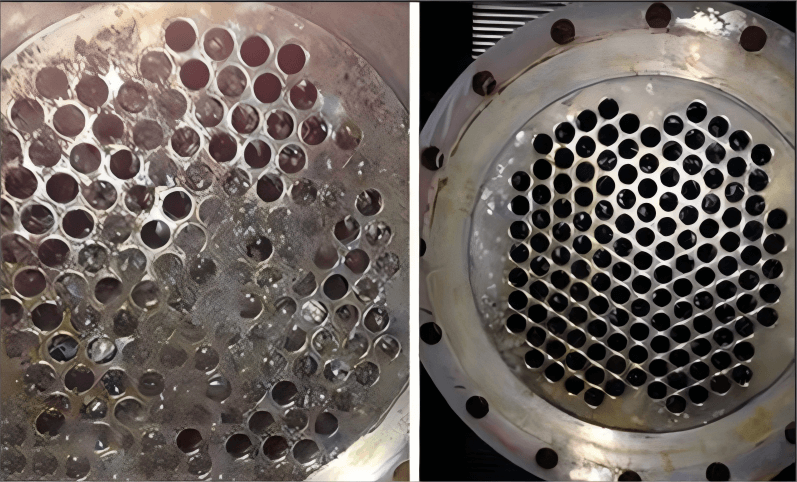
I. What’s the scale?
Currently, with the ZLD crystallization resource utilization marking a development trend for high-salinity wastewater treatment, thermal evaporation methods, such as MVR evaporation, and MEE evaporation, are widely adopted.
However, the high-salinity industrial wastewater characteristics such as high salinity, hardness, and silicon content and the presence of complex organic substances have a significant impact on the heater:
Ions like Ca²⁺ and Mg²⁺ in the water can easily combine with carbonates to form scale on the solution contact surface in the heat exchanger, resulting in a great efficiency reduction on the treatment device and heat exchanger.
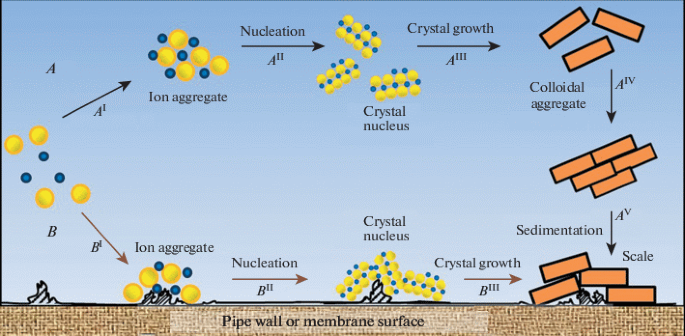
II. How does the scale form inside the tube?
It involves the following elements during the scale formation:
- Shape and roughness of the heat tube wall,
- Fluid speed
- Difference in heat transfer temperature
- Difference in heat exchange tube pressure
- Shape and roughness of the heat tube wall
The shape and roughness of the heat tube wall affect the scale precipitation process directly. A rougher heat exchange tube wall will reduce the scaling period, and increase the scaling speed dramatically.
While there is a turbulent flow in the heat exchange tube, the wall’s roughness also affects the flow resistance, which results in an increase in energy consumption.
- Fluid speed
The effluent flow speed in the heat exchange tube is a key element affecting the scale growth.
Due to the hydrokinetic force, the scale sedimentation speed has been strongly reduced, and the formation of the attached scale has been restrained. But, the increase of the flow rate facilitates the particle transport efficiency, resulting in an accelerated scaling speed with the mounting amount of adhesion scale.
According to the project experience, it is found that the flow rate exhibits a non-monotonic relationship with the formation of the mixed scale. Adopting the flow rate within a reasonable range economically will reduce the scale formation rate and the energy consumption most effectively.
- Difference in heat transfer temperature
In the evaporation design, an overrated heat transfer temperature difference causes local overheating, which might lead to crystallised particles forming on a scale during the disposal of the effluent containing salt.
Overrated heat difference occurring on both sides of the tube will cause a massive temperature gap between the heat exchange wall and the solution, followed by scale formation, accelerated material denaturation, and increasing deposit scale amount.
- Difference in heat exchange tube pressure
Due to the existence of stagnant bad in the heat exchange tube of the heating cabin, the effluent close to the tube wall contains a higher temperature than its counterpart flowing in the centre of the tube. According to relevant documents, the average of such temperature difference reaches about 3 ℃.
With low static pressure difference and insufficient liquid height in the heat exchange tube, if the average temperature of the effluent stays below the boiling point, the liquid staying close to the tube wall might have already reached the boiling state.
This results in an increase in salt concentration and crystallisation at the stagnant bed.
With the appearance of the scale formation, the heat exchange efficiency in the heat chamber starts getting worse, overloading the stagnant bed’s temperature. Such circulation results in a vicious situation until the tube is blocked.
III. How does the scale impact the devices negatively?
- Constantly increase the device’s energy consumption,
- Constantly reduce the heat transfer coefficient,
- Constantly reduce the effective steam volume by occupying the tube space,
- Constantly reduce the product efficiency of the whole system,
- Block the heating tube, resulting in the shutdown of the whole system,
- Cut the service lifetime of the device.
IV. What kind of scale will we encounter?
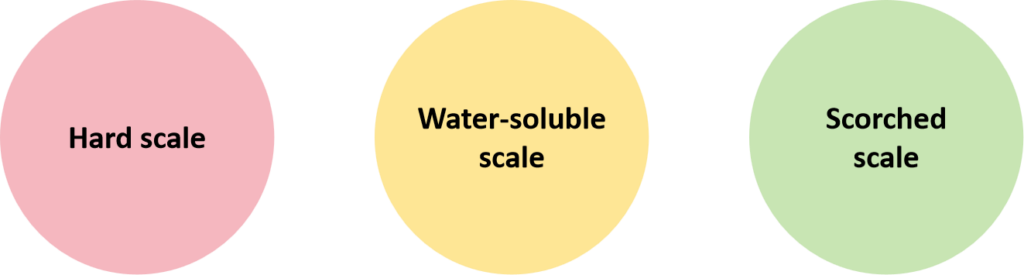
- Hard scale:
Mainly refers to the insoluble inorganic salt formed by Ca2+ and Mg2+ ions, known as the ordinary scale.
- Water-soluble scale:
Mainly refers to the inorganic salt soluble in water, which will precipitate and attach to the heat exchanger during the process. Such as sodium sulfate(Na2SO4) and sodium carbonate(Na2CO3). Such a scale crystallises from a supersaturated solution when its concentration exceeds the solubility limit.
- Scorched scale:
Refers to the scorched solute, mostly black. On the one hand, its formation is caused by local overheating triggered by the vapour bubbles on the heat exchanger surface; moreover, it is caused by the uneven distribution of the liquid and flash evaporation, which leads to a swift process resulting in dry-out or overconcentration.
V. What solutions can we offer in dealing with the scale?
- Prevent method
- Pretreatment
By effluent filtration or softening to remove any material that could cause scaling in advance.
- Water quality control
Maintain the effluent quality at a proper Hp vale and add a scale inhibitor as preventive.
- Regular cleaning
Clean the heat exchange tube on a regular basis before massive concentration scale formation.
- Optimise the operation parameter
Control the device’s operation temperature, pressure, and flow rate to avoid local overheating occurrences, to reduce scale formation.
- Removal method
Due to different situations, the scale can be removed with or without shutting down the evaporation system
- Water dissolving;
Adopting massive amounts of water, through continuous soaking and washing, only reaches a limited result.
- Chemical descaling
Add the acidic, alkaline, or special cleaning agent through continuous soaking, softening, and washing to remove the scale.
- Mechanical descaling;
Adopt the brush, scraper, or even high-pressure water jet to break and remove the high-concentration scale.
Combining countless project programs and research innovations, our group specialises in evaporation and salt separation treatment for different types of high-salt and high-COD effluent containing solutes such as NaCl, Na2SO4, (NH4)2SO2, NH4Cl, or KCl. By adopting different technical combinations, we’re dedicated to providing our clients with satisfactory service, to serve the alive program conditions.
Fields we are involved in: coal chemical industry, mine water, coking, hazardous waste, fine chemical industry, steel, electric power, and new energy.
We hold the idea of introducing innovation with an elaboration study and manufacturing with craftsmanship as our true belief, in our path of becoming a respectable service provider in the zero-discharge & recycling business.